A compilation of some of the practical exercises conducted in our laboratory. Exercises may vary across institutions but some are common.
Introduction: Microorganisms are so called because they are so small that they can not be ordinarily seen using unaided eye. The optical instrument that magnifies the image of these organisms that enables us to view their morphological features is a microscope. Antony van Leeuwenhoek is often considered as the father of microscopy, although compound microscopes were actually invented much before. A compound microscope contains several sets of lenses that magnify the image at different levels. Typically, the image is magnified initially by the objective lens and then again by the eye piece before it reaches the eye.
Most bacteria measure in the range of 0.5 to 4 µm (micrometer NOT microns). Mycoplasma and Coxiella are the shortest among bacteria and Spirochetes the longest. Viruses are ultramicroscopic structures; they can’t be seen by compound microscopes. They can be visualized by electron microscopes and their measurements are in nm (nanometer). The Å (Armstrong units) is the unit of measurement for still smaller particles.
1 mm = 1000 µm
1 µm = 1000 nm
1 nm = 10 Å
Various types of microscopes are:
- Simple (dissection) microscope
- Compound microscope
- Darkground microscope
- Phase contrast microscope
- Fluorescent microscope
- Interference microscope
- Electron microscope
- Atomic Force microscope
- Polarization microscope
Principles of microscopy and concepts
Magnification:
This represents the number of times the image of a
specimen is amplified. 10x means the size of the image is increased by ten
times. The magnifying power of the lens is limited. After a certain point
the magnification results in a blurred image and is termed empty
magnification. Even with the best optics, 1400x is the highest useful
magnification achieved. Magnifying power of an objective is determined by
the dividing the optical tube length by the focal length of the lens.
Optical tube length is the length of the microscope body tube between the
nosepiece opening, where the objective is mounted, and the top edge of the
observation tubes where the eyepieces are inserted. In most microscopes, it
is fixed at 160mm.
Low power dry objective: 160/16 = 10x
High power dry objective: 160/4 = 40x
Oil immersion objective: 160/1.7 = 94x or approximately 100x
Numerical Aperture:
The numerical aperture of the lens is an
important consideration in optics as it dictates the angle at which the
light enters it. The light-gathering ability of a microscope objective is
quantitatively expressed in terms of the numerical aperture. Higher values
of numerical aperture allow increasingly oblique rays to enter the objective
front lens, producing a more highly resolved image.
It is defined by the following formula: Numerical Aperture (NA) = n × sin(θ)where n is the refractive index of the medium between the object and the objective θ is one-half the angular aperture (angle of aperture is the angle formed by the two most divergent rays of light which enter the objective, starting from the center of the object).
Refractive index of oil is 1.5 and that of air is 1.0.
NA of dry objective: 1 x sin 90° = 1, but practically the highest practical
numerical aperture of a dry lens is 0.95.
NA of oil immersion objective: 1.5 x sin 90° = 1.5, however in practice only
1.4 is achieved for apochromatic objective and 1.3 for achromatic objective.
Limit of resolution or resolving power: In simple words, it is the ability to see two closely placed dots as two separate dots. If the distance between the two points is lessened, it would appear as a single point. It is expressed quantitatively as limit of resolution. The resolution of human unaided eye is 200 µm. This means that human eye can not see objects small than 200 µm. The resolving power of compound microscope is 0.2 µm and that of electron microscope is 1-10 nm. The limit of resolution depends on the wavelength of the light used. Resolution increases with the decreasing wavelength of light. Violet colour light offers more resolution that red coloured light. Electron beams, which have very low wavelength offers maximum resolution. It is calculated by using formula:
LR = 0.61 x wavelength of light
Numerical
aperture
For example, if green light of wavelength 0.55 µm is used and oil immersion objective with NA 1.4 is used, the maximum resolution obtained is 0.24 µm
0.61 x 0.55 = 0.24 µm1.4
Definition:
This is the capacity of the objective to render the
outline of the image clear and distinct. Definition of an image is disturbed
by spherical or chromatic aberrations. The central part of the image is
usually well focused but the edges may suffer some aberration, which are of
two types; spherical or chromatic. In spherical aberration, the periphery of
the image appears out of focus. This happens because all the light passing
through the lens doesn’t condense at the same point. In chromatic
aberration, the light is split into different colours at the peripheral part
of the image since the edges of the lens act like a prism. The aberrations
can be corrected by using achromatic or apochromatic lenses.
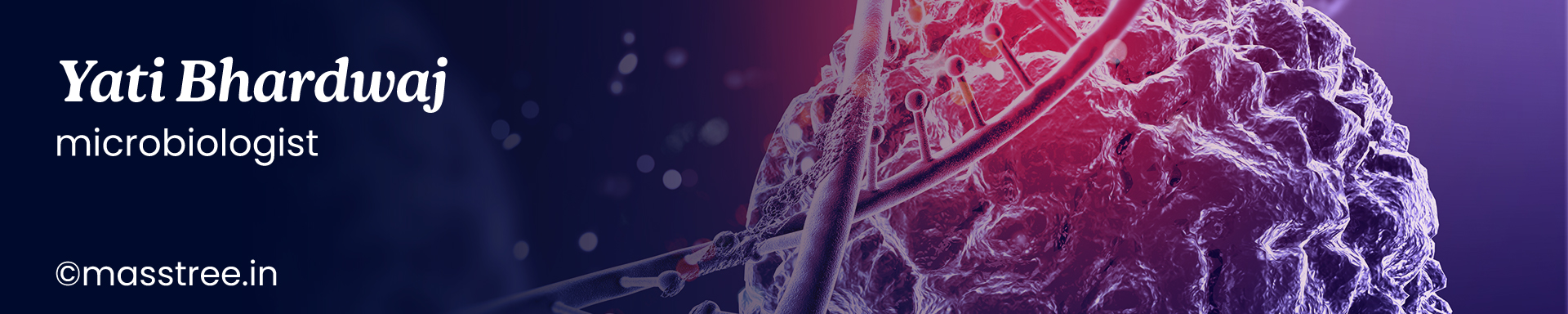
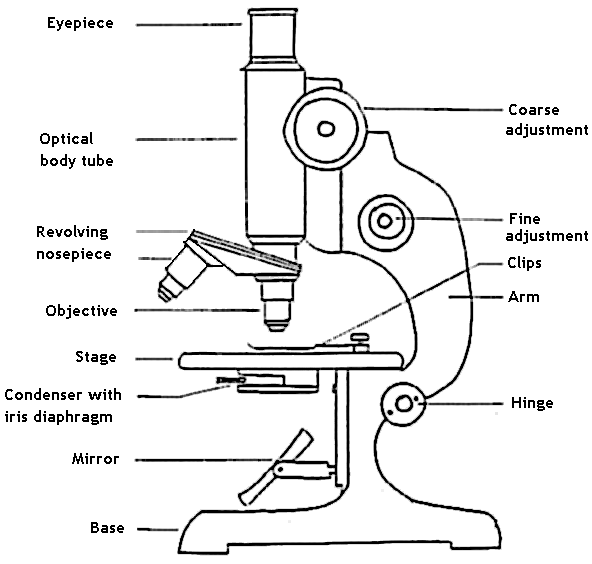
Parts of (student's) compound microscope:
Source of light: Brightfield microscope uses visible light for illumination; specimen appears against a bright background. In brightfield microscope, the source of light is sunlight or indoor bulbs, or inbuilt lamps. The inbuilt lamps are made up of low voltage tungsten filament lamp or more recently LED lamp.
Condenser: The parallel beam of light from natural or artificial source is condensed into a cone of light that illuminates the specimen or the object (smear on slide) by the sub-stage condenser. If the source of light is sunlight or indoor bulbs, the light is diverted to the condenser using the mirror. Concave mirror is used when low or high power objective lenses are used, whereas plane mirror is used when oil immersion objective lens is used. Mirrors are absent when in-built lamps are used. The condenser consists of series of lenses, which focuses the light to the object placed on the stage. Kohler illumination ensures that the diffuse light of uniform brightness is available without the view of the source of light. Various types of condenser in use are Abbe, Paraboloid and Cardoid condensers. The Abbe condenser (which is named after its inventor Ernst Karl Abbe) is the simplest of condensers that contains two lenses. The condenser can be lowered or raised according to the requirements. The condenser is lowered while using low power dry objectives, and raised while using oil immersion objectives. The amount of light passing on to the specimen from the condenser is regulated by using iris diaphragm. Light is reduced by closing the diaphragm partially for use with dry objectives. Oil immersion objectives require maximum light and this is achieved by keeping the iris diaphragm fully open.
Objective lens: The light passing through the object (specimen) enters the objective lens. Typically, most microscopes have four objective lenses mounted on a revolving nose piece. The dry objectives include scanner (5x), lower power (10x) and high power (40 or 45 x). The oil immersion objective (100x) offers maximum magnification.
Eyepiece: Depending on the type of microscope, the magnified image may travel straight or can be reflected or divided using prism towards the eye piece. Monocular microscopes have single eyepiece where binocular microscopes have two. The magnified image is again magnified in the eyepiece lens and an inverted image is formed on the retina of the viewer’s eye. Older Huyghenian eye pieces were used with achromatic objective lenses but the newer apochromatic objectives require compensating eye pieces. These eye pieces correct the lateral colour errors of their objectives. In a compound microscope, the image is magnified twice; first by the objective lens and then by the eye piece. The eye piece consists of two planoconvex lenses with a circular diaphragm between them. Their magnification can vary form 5x to 10x.
The magnifications of the image in a compound microscope are as follows:
| Objective lens | Eye piece | Total magnification |
|---|---|---|
| 5x | 10x | 50x |
| 10x | 10x | 100x |
| 40x | 10x | 400x |
| 100x | 10x | 1000x |
The image of a specimen is magnified approximately 1000 times using a compound microscope. With an inclined body, the magnification of the microscope may be improved by another 1.5 times. With the highest practically achievable NA of about 1.4, 1400x is the highest useful magnification that can be achieved.
Since the condenser is fully raised for use with oil immersion objectives, light focused on to the specimen are at acute angles. For this reason the objective lens must be as low as possible. Despite this, the light can pass away without entering the objective lens due to diffraction suffered by light when moving from one medium to another (glass to air). Hence, the place between the glass slide and oil immersion objective is filled with immersion oil having same refractive index as that of glass. The light rays leaving the glass do not deviate and enter straight into the objective lens.
Even though the magnification of 1000x is achieved using 100x objective of 1.4 NA and 10x eyepiece, it does not provide image as sharp as that obtained with magnification of 400x using 40x objective of 0.65 NA and 10x eyepiece.
Adjustments for dry objectives (5x, 10x, 40x):
Mirror: concave, condenser: partially lowered, iris diaphragm: partially closedAdjustments for oil immersion objective (100x):
Mirror: plane, condenser: fully raised, iris diaphragm: fully open
Focusing the slide:
Before viewing the slide under microscope, it is important to obtain a
bright background. For oil immersion objective usage, follow these steps:
Ensure that iris diaphragm is fully open and condenser fully risen. With 10x objective in place, look through the eyepiece and adjust the plane mirror such a way that you obtain perfectly bright and uniformly lit white background.
Place a drop of oil on the stained smear and place it on the stage and center it such a way that the smear is above the source of light and below the objective lens.
Shift to 100x objective and raise the stage till the oil on the slide touches the objective. This must be done looking sideways and not while looking through the eyepiece or else the slide will break and damage the objective lens.
Using fine adjustment, try to focus the image. Move the slide accordingly to obtain a good field.
After viewing and noting down the observations, lower the stage and remove the slide.
Proper usage and handling the microscope:
Microscope is a delicate instrument that must be handled with care. All kinds of mechanical shocks must be avoided. The microscope must be lifted by holding its arm in one hard and supporting the base of the microscope with the palm of the other hand. The microscope must be kept in dust free environment. The oil must be wiped clean using a soft tissue paper. After usage the slide must be removed and cleaned before returning to its original place.
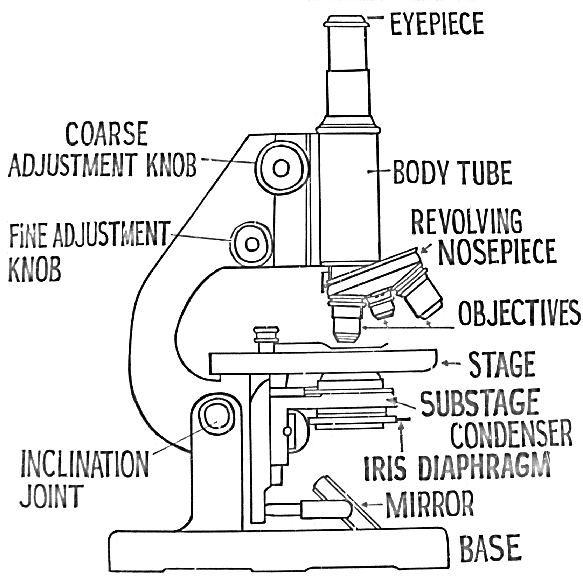
Darkground (darkfield) Microscopy:
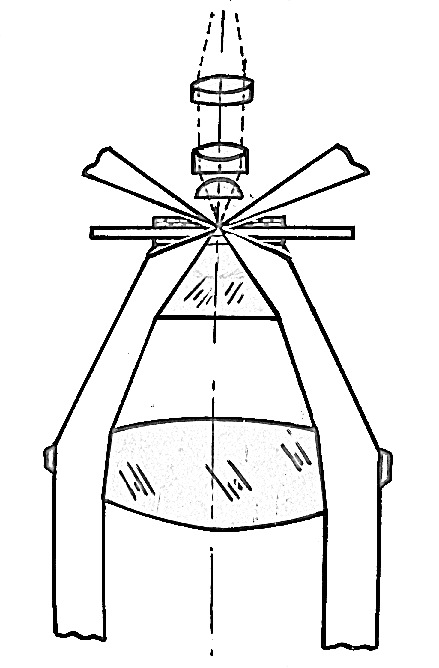
Slender bacteria such as spirochetes (e.g. Treponema, Borrelia) are difficult to visualize under compound microscope using brightfield illumination. In order to visualize such bacteria, darkground microscopy is employed. Here, the microbe is made to appear brightly lit against a dark background. In order to achieve this, a special darkfield condenser is used that focuses a hollow cone of light on the specimen. An opaque disc is often placed in the center of the condenser to produce a hollow cone of light. The light rays are directed such a way that they don’t enter the objective directly, hence the background is dark. Only those rays of light that are reflected by the organism enter the objective, hence they appear bright against a dark background. In more sophisticated darkfield condensers (such as paraboloid and cardioid), the occlusion of the direct light and the utilization of oblique rays are achieved by use of specially designed mirror surfaces instead of opaque disc. Darkfield microscopy requires a bright/intense source of illumination. Darkfield microscopy can also be used for observing bacterial motility.
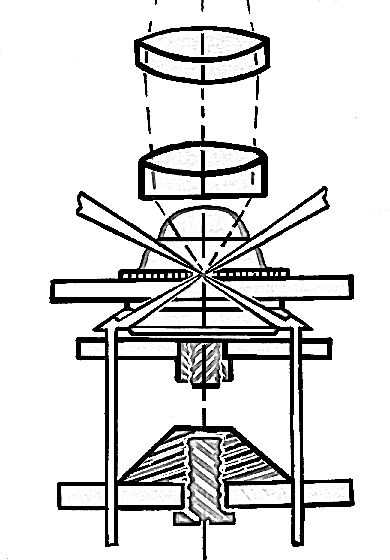
Pathway of light through Abbe condenser
Pathway of light through Paraboloid condener
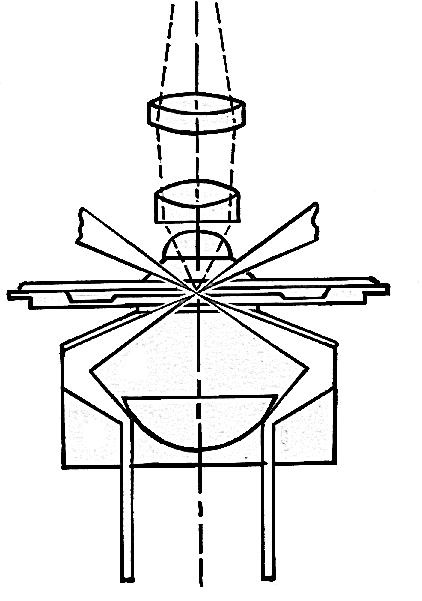
Pathway of light through Cardioid condenser
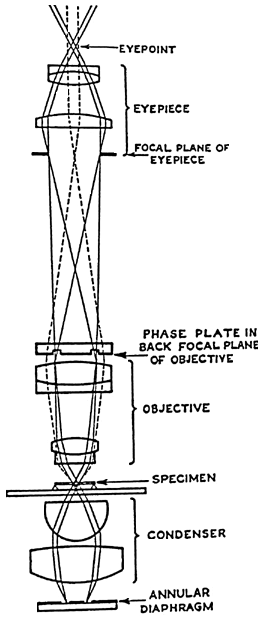
Phase contrast microscopy:
Unstained preparation such as saline wet mount is often used to demonstrate living microorganisms and their motility. Visualization of their morphology and internal structures is difficult because they don’t have colour of their own and contrast poorly with the background. Phase contrast microscope is used to create contrast between the organism, its structures and the background, thus making its visibility quite clear.
Principle: Phase contrast microscopy is suitable for unstained preparation. A specimen may contain thin and thick areas. Using an annular diaphragm (phase annulus), a hollow cone of light is focused on the specimen. When light travels through thin area, it passes undeviated but when it passes through a thick area, it gets deviated. Such a deviated light is said to have slowed down or out of phase by ¼ of wavelength.
This difference is too little for human eye to appreciate; hence the difference is magnified by letting the light rays to pass through a phase plate. A phase plate is placed at the back focal plane of the objective lens. Undeviated rays pass through thin area of the phase plate whereas the deviated rays pass through thick area of the phase plate.
Thus, the deviated rays are slowed down again or out of phase by ¼ of wavelength. The undeviated and deviated light rays are now out of sync by ½ wavelength and when these rays meet, they result in destructive interference. The difference of ½ wavelength is sufficient for human eye to perceive, and the thick objects appear darker and the thinner objects appear lighter.
Electron microscopy:
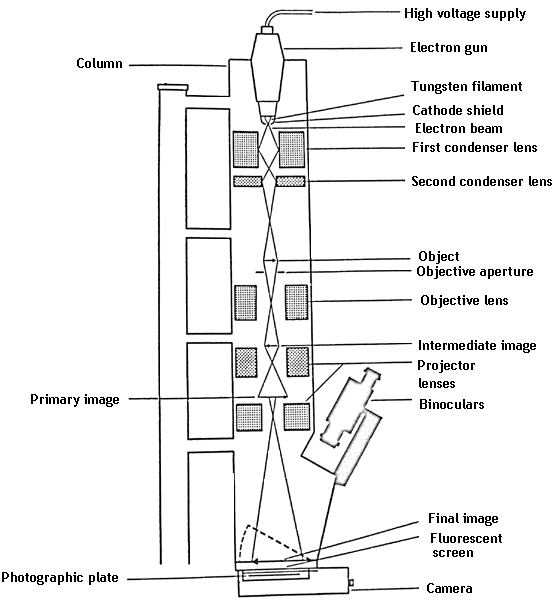
Resolution of a magnifying system can be improved by decreasing the wavelength of light. Since the wavelength of light is limited, electron beams of wavelength can be used to magnify images up to 1,00,000 times. Depending on the type of electron microscope a resolution of 1-10 nm can be achieved.
Electron microscope works on a principle similar to that of compound microscope only that electron beams replace light and electromagnets replace the optical lenses. A high voltage electron gun is used to produce a beam of electrons. Since air can scatter electrons, the chamber of EM is a vacuum. As electrons have poor penetrative power the specimen must be very thin.
Electron beams are focused by means of a pair of condenser lenses on the specimen. The resulting image is produced by an objective lens, which is then projected by a pair of projector lenses. Since human eye is not sensitive to image formed by electron beams, the image is formed on a fluorescent screen.
Since the specimen is kept in perfect vacuum, live specimens can't be viewed. The image generated is actually a shadow of the specimen and colour image is not produced.
There are two types of electron microscopes: transmission electron microscopes (TEM) and scanning electron microscopes (SEM).
Introduction: Some bacteria are motile and some other are non-motile. Motile bacteria usually use flagella as their locomotory organ. Bacteria tend to move towards or away from various chemotactic, phototactic, aerotactic or magnetotactic stimuli. Description of bacterial flagella is available here. There are various ways to demonstrate bacterial motility. These include, a simple wet mount, hanging drop preparation, or employment of soft gels (semi-solid agar).
Requirements for hanging drop preparation: Fresh broth culture of bacteria, bunsen burner/spirit lamp, bacteriological loop, glass slide (with central concavity/paraffin ring/adhesive ring), cover slips and microscope.
Procedure: A loopful of bacterial suspension is placed in the center of a cover slip. The glass side with stick ring is placed over the coverslip such a way that coverslip sticks to the ring on the slide. Immediately, the glass slide is lifted and turned around. The drop of bacterial suspension now "hangs" on the lower surface of the coverslip. The drop is then observed under the low power (10x) dry objective of the compound microscope. The edge of the drop must be focused. Bacteria tend to accumulate at the edge of the drop. Once the edge is located, it is then observed under 40x high power objective.
Observation under 10x objective:
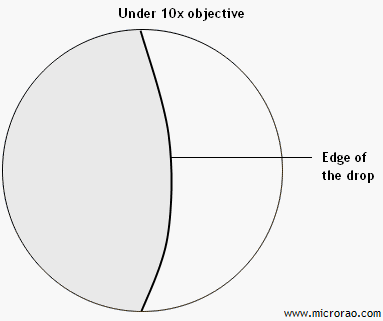
Edge of the drop is seen.
Observation under 40x objective:
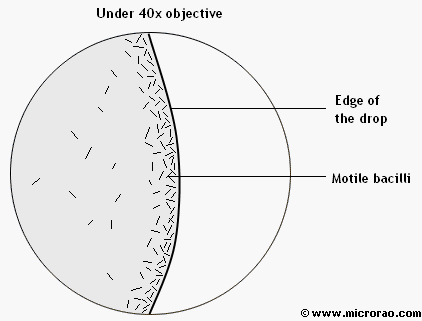
Motile bacilli are seen at the edge of the drop.
Introduction: Bacteria are microscopic organisms that can not be seen with unaided eye. They can be seen even in unstained preparations such as a wet mount or hanging drop preparation but the morphology is not clear. Bacteria are colorless and when suspended in saline they don’t offer any contrast. Besides, bacterial motility makes it difficult to observe the morphology clearly. Hence, bacteria have to be stained to observe them. The dyes often used are toxic chemicals that kill the bacteria. The process of smearing, fixing and drying often kill the bacteria. This process fixes the bacteria to the slide and their position on slide remains unaltered.
Preparation of smear:
A grease free glass slide is taken and a circle is marked one side of
the slide
using a wax pencil or a glass marker pen. Marking the slide makes it
convenient
to identify the surface of the slide that contains the smear. On the
opposite
side, a small drop of sterile saline is taken. Saline may also be
transferred
using a bacteriological loop. A small portion of the colony is picked up
using a
sterile straight wire and emulsified well in the saline on the slide.
The colony
is spread evenly within the circled are to produce a smear. Presence of
grease
on the slide would prevent uniform distribution resulting in an uneven
smear.
Taking too much of the colony would result in excessively thick smear.
Similarly, taking a very miniscule part of the colony make it very
difficult to
look for bacteria. Once a uniform smear is prepared, it has to be air
dried. The
smear must be held above the Bunsen flame at comfortable height. The
smear must
be not be forcibly dried by applying heat. After the smear dries, it is
fixed.
Smears can be fixed physically or chemically. Chemical fixatives are not
useful
for regular bacteriological studies. It is convenient to heat-fix the
smear by
gently passing the slide through the Bunsen flame once or twice.
Excessive
heating of the slide must be strictly avoided.
Note: You don't have to prepare smears, slides containing smear would be provided to you in the practical class.
Requirements:
Smears on glass slide, staining rack, staining solutions, blotting
paper,
immersion oil and microscope.
Staining:
There are several dyes that can be used to stain the bacteria. Simple
staining
technique utilizes single basic dye such as crystal violet, methylene
blue,
basic fuchsin etc. All bacteria take up the basic dye uniformly and
appear in
the same colour. Only the morphology of the bacteria can be appreciated
upon
staining.
We use two simple staining solutions, namely crystal violet solution and dilute carbol fuchsin solution. We use the former to demonstrate cocci and the latter to demonstrate bacilli. This choice is not compulsory but is used by convention. The slide with cocci in the smear would be market ‘C’ and the one with bacilli would be marked ‘B’ only to avoid mixing up the two slides.
The slide marked ‘C’ is placed on the staining rack above the sink. Few drops of crystal violet solution is poured over the smear enough to cover the circled area. Care must be taken not to flood the slide, which would be a wastage of staining solution. The stain must be allowed to remain on the smear for a minute. The slide is then lifted from the rack, tilted to run the stain down to the sink and then washed under gentle stream of running tap water. A washing bottle may also be used for the same purpose. The slide must be held at inclined position and a thin stream of water must fall on the slide a little above the smear. Water must not be allowed to fall directly on the smear as there are chances of the entire smear getting washed away. Once the smear is sufficiently washed, it is placed on a piece of blotting paper. The sides of the paper are then folded over the slide and gentle pressure is applied to dry the slide. Excessive pressure or wiping the paper over the slide may completely wipe off the smear.
The procedure for staining the slide marked ‘B’ is similar to the procedure mentioned above except that the staining solution to be used is dilute carbol fuchsin and the time of contact is only 30 seconds.
After the slide is dry, a drop of cedar wood oil (or liquid paraffin) is placed on the smear. The slide is placed on the mechanical stage and smear portion is centered above the condenser. The microscope is adjusted for viewing under oil immersion objective (100x) by raising the condenser fully, opening the diaphragm fully and using the plane mirror. This ensures that maximum light is available. The stage is raised (or optical body is lowered; depending on the type of microscope) using coarse adjustment till the oil immersion objective touches the oil. It is subsequently focused using fine adjustment until a clear image appears. The small circular area of smear that is seen through the eyepiece is called a field.
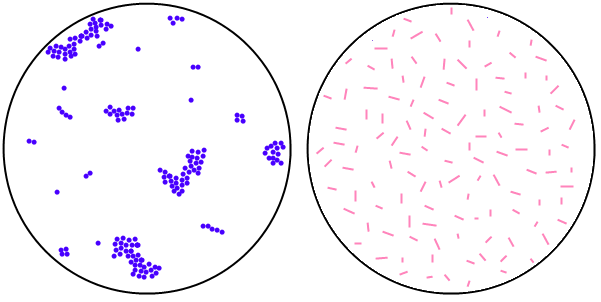
Observation for slide marked ‘C’ stained by crystal violet
solution:
Violet coloured, spherical shaped bacteria that are arranged in singles,
pairs,
tetrads, short chains and irregular grape like clusters are seen.
Inference: The given slide contains one of the cocci.
Observation for slide marked ‘B’ stained by dilute carbol fuchsin
solution:
Pink coloured, rod shaped bacteria that are present haphazardly (without
any
characteristic arrangement) are seen.
Inference: The given slide contains one of the bacilli.
Note: a. Observation must be made in the following order: colour >
shape >
arrangement
b. Spherical shaped bacteria are referred as cocci (coccus is
singular) and
rod shaped bacteria as bacilli (bacillus is singular)
b. This exercise is not included in the practical examination, it is
only
for your learning.
For more information on Frequently asked question in Staining techniques, click here
Introduction: Bacteria vary in certain physiological and biological properties. Some staining techniques utilize these differences to stain the bacteria differently. Such staining methods are called differential staining methods, these include Gram staining and acid fast staining. Different bacteria stain differently to a common staining procedure. Gram stain was described by Danish bacteriologist Hans Christian Gram in 1884 to differentiate Streptococcus pneumoniae from Klebsiella pneumoniae in lung tissue. The original formulation comprised of aniline gentian violet, Lugol's iodine, absolute alcohol and Bismarck brown.
Principle of Gram staining technique:
There are four components of Gram stain; primary stain, mordant,
decolorizer, and counterstain. When stained with a primary stain and
fixed
by a mordant, some bacteria are able to retain the primary while others
get
decolorized by a decolorizer. Those bacteria that resist decolorization
and
retain the primary dye are called Gram positive and those bacteria that
gets
decolorized and then get counterstained are called Gram negative.
Various
theories have been put forth to explain the differences among bacteria
that
are exploited by this staining technique; some are more acceptable than
others.
Typically, gram positive bacteria have thicker cell walls (approximately 40 layers of peptidoglycan) compared to Gram negative bacteria, which have only 1-2 layers of peptidoglycan.
Alcohol or acetone, which is used as a decolorizer dissolves lipids in the membranes; since Gram negative bacteria have an additional membrane (apart from cytoplasmic membrane), more lipids are lost forming pores through which the dye-iodine complex escapes during decolorization. On the other hand, alcohol dehydrates the Gram positive bacteria closing the pores and shrinking the cell wall and thus prevents outflow of dye-iodine complex.
Slightly lower cytoplasmic pH (2.0) in Gram positive bacteria helps the basic dye to bind more efficiently than the Gram negative bacteria who have slightly elevated pH (3.0). This theory is probably not widely accepted.
Another theory such as presence of Magnesium ribonucleate in Gram positive bacteria and its absence in Gram negative bacteria has not received widespread acceptance.
At the end of Gram staining, Gram positive bacteria appear violet or purple whereas Gram negative bacteria appear pink.
Preparation of smear:
A grease free glass slide is taken and a circle is marked one side of
the
slide using a wax pencil or a glass marker pen. Marking the slide makes
it
convenient to identify the surface of the slide that contains the smear.
On
the opposite side, a small drop of sterile saline is taken. Saline may
also
be transferred using a bacteriological loop. A small portion of the
colony
is picked up using a sterile straight wire and emulsified well in the
saline
on the slide. The colony is spread evenly within the circled are to
produce
a smear. Presence of grease on the slide would prevent uniform
distribution
resulting in an uneven smear. Taking too much of the colony would result
in
excessively thick smear. Similarly, taking a very miniscule part of the
colony make it very difficult to look for bacteria. Once a uniform smear
is
prepared, it has to be air dried. The smear must be held above the
Bunsen
flame at comfortable height. The smear must be not be forcibly dried by
applying heat. After the smear dries, it is fixed. Smears can be fixed
physically or chemically. Chemical fixatives are not useful for regular
bacteriological studies. It is convenient to heat-fix the smear by
gently
passing the slide through the Bunsen flame once or twice. Excessive
heating
of the slide must be strictly avoided.
Note: You don't have to prepare smears, slides containing smear would be provided to you in the practical class.
Staining procedure:
The glass slide with smear facing upwards is placed on the staining
rack.
Few drops of crystal violet solution are poured over the smear taking
care
not to extend beyond the circled area. The stain is allowed to act for
one
minute. Gentian violet and Methyl violet are the other alternatives for
primary stain. The slide is washed in gentle stream of running tap
water.
Few drops of Gram's iodine is placed over the smear and allowed to act
for
one minute, after which it washed in tap water. The smear is decolorized
using alcohol-acetone decolorizer by holding the slide in an inclined
position and pouring the decolorizer from the top end. As the
decolorizer
flows over the smear, it decolorizes it. When the decolorization is
complete, the fluid that flows over would be colorless. The entire
decolorization process should be completed within 30 seconds. Most
errors in
staining occur at this stage. Decolorization for excessive duration is
called over-decolorization wherein Gram positive bacteria appear Gram
negative. Similarly, rapid decolorization may lead to
under-decolorization
of smear wherein Gram negative bacteria appear Gram positive. Absolute
alcohol, acetone or a mixture of alochol and acetone (1:1) are used as
decolorizer. After decolorization the smear is washed and counterstained
by
dilute carbol fuchsin for 30 seconds. Other alternatives include
safranin
and neutral red. The slide is dried using blotting paperr, a drop of oil
is
placed on the smear and observed under 100x (oil immersion) objective.
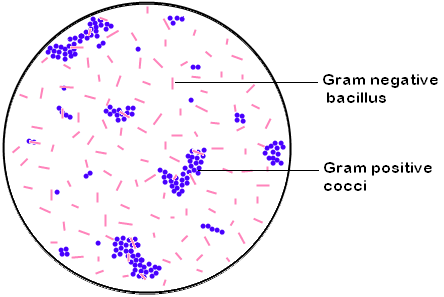
Observation:
Violet coloured, spherical shaped bacteria in
singles, pairs, tetrads, short chains and irregular grape like clusters
seen
along with pink colored, rod shaped bacteria that are present
haphazardly.
Inference:
The given smear contain both the Gram positive
cocci
and the Gram negative bacilli.
Note: a. Observation must be made in the following order: colour >
shape >
arrangement
b. Spherical shaped bacteria are referred as cocci (coccus is
singular)
and rod shaped bacteria as bacilli (bacillus is singular)
c. While we provide a slide containing both Gram positive cocci and
Gram
negative bacilli in the smear for routine practical classes, you may
get
slides containing either of them alone or a mixture during your
examination.
For more information on Frequently asked question in Staining techniques, click here
Introduction: The cell wall of Mycobacterium sps typically contain waxy substance (mycolic acid) that makes it impervious to staining by aqueous staining solutions. These bacteria can not be stained by simple stains or even by Gram staining. These bacteria can however be stained by drastic measures, and once stained can not be readily decolourized by weak mineral acids. Hence, these bacteria are called acid fast bacilli and the staining method is called acid fast staining. It is also a type of differential staining method.
Acid fast staining was introduced by Ehrlich in 1882. It was subsequently modified by Ziehl and Neelsen. There are two types of acid fast staining: hot method and cold method. Ziehl-Neelsen is a hot method of acid fast staining. The components of Ziehl-Neelsen stain include primary stain (strong/concentrated carbol fuchsin), decolourizer (20% H2SO4) and counterstain (Loeffler's methylene blue).
Strong carbol fuchsin solution is basic fuchsin dissolved in phenol (carbolic acid). Heating the slide helps to soften the waxy material on the bacterial cell wall. The waxy material is hydrophobic to aqueous solution but not to phenolic solution of basic fuchsin. Hence strong carbol fuchsin is able to stain the cell. Upon staining, they tend to resist decolourization by 20% H2SO4 (sulphuric acid). The background is then stained by Loeffler's methylene blue.
One of the main applications of acid fast staining is the diagnosis of pulmonary tuberculosis. Sputum sample obtained from the patient is used to make thin and uniform smear on glass slide. The readymade smear is provided to you.
Requirements: Smear on glass slide, staining rack, spirit lamp, Strong carbol fuchsin, 20% H2SO4, Loeffler's methylene blue, blotting paper, immersion oil, microscope.
Procedure:
Keep the slide with the smear facing upwards on the
staining rack. Flood the entire slide with strong carbol fuchsin
solution.
Heat the slide with gentle waving from below using lighted spirit lamp
until
fumes arise from the stain. Do not let the stain solution boil or dry
out.
Pour more stain if required. Allow the fumes to disappear and repeat the
process of heating the slide two more times. Allow the slide to cool
down.
Pour off the stain and decolorize the smear using 20% H2SO4 for at least
one
minute. Wash the slide under gentle stream of running tap water. Examine
the
slide for signs of proper decolourization. The smear must be almost
colourless or faintly pink. Repeat the process of decolourization until
the
smear get properly decolourized. Wash the smear and place the slide back
on
the rack. Clean the other side of the slide with 20% H2SO4, if it is
stained
as well. Cover the smear (NOT the entire slide) with few drops of
Loeffler's
methylene blue and allow it to act for a minute. Was the slide in tap
water
and dry it using blotting paper. Place a drop of immersion oil on the
smear
and observe it under 100x objective.
Observation:
Pink coloured, slightly curved bacilli in singles
or
in small clumps seen against a blue background of epithelial cells and
pus
cells.
Inference:
The given smear contains one of the acid fast
bacilli
(May be Mycobacterium tuberculosis).
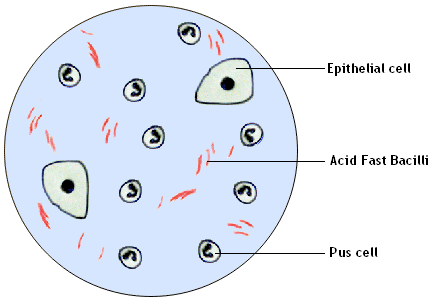
Note: You will have search many fields to spot Acid Fast Bacilli.
For more information on Frequently asked question in Staining techniques, click here
Introduction: Albert's staining technique is a type of special staining technique since it is used to demonstrate a special structure in bacteria. It is chiefly used to demonstrate metachromatic granules found in Corynebacterium diphtheriae. This bacterium is responsible for the disease diphtheria. The name Corynebacterium is derived from the Greek word "Coryne", which refers to the club shape of the bacteria seen in old cultures. The storage granules in this bacterium is called metachromatic granules because it exhibits the property of metachromasia, wherein the granules appear in a colour other than the colour used for staining. When stained with polychrome methylene blue, the granules appear violet while the rest of the bacillus appears blue. The granules are made up of polymetaphosphates and are known by various other names such as volutin bodies, Babe-Ernst granules or polar bodies. The bacterium produces the granules in abundance when grown on nutrient rich medium such as Loeffler's serum slope.
When stained with Albert's stain, the bacillus stains green whereas the granules stain bluish black. There are two reagents that are used in the staining process: Albert's A solution and Albert's B solution. Albert's A solution consists of Toluidine blue, malachite green, glacial acetic acid, and ethyl alcohol. Albert's B solution contains Iodine and Potassium iodide in water.
Requirements: Smear on glass slide, staining rack, Albert's A solution , Albert's B solution, blotting paper, immersion oil, and microscope.
Procedure:
Place the slide on the staining rack with smear
facing
upwards. Cover the smear with Albert's A solution and allow it to act
for 7
minutes. Pour off the stain and wash the slide in Albert's B solution
(NOT tap
water). Place the slide back on the rack and cover the smear with
Albert's B
solution and let it act for two minutes. Pour off the stain and wash the
slide
in tap water. Dry the slide using blotting paper, place a drop of
immersion oil
on the smear and observe under oil immersion objective.
Observation:
Green coloured, rod shaped bacteria that are
arranged at
angles to each other resembling English letter 'L', 'V' or Chinese
letter
pattern along with bluish black metachromatic granules at the poles
seen.
Inference:
The given smear contains bacteria morphologically
resembling Corynebacterium diphtheriae.
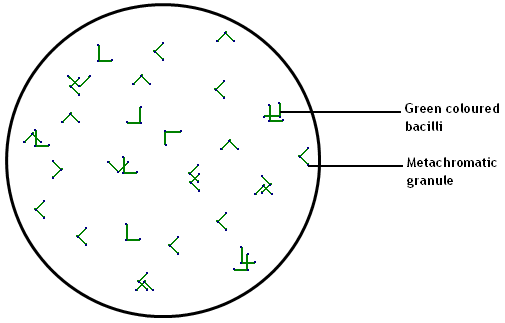
Note: You will have search many fields to spot Acid Fast Bacilli.
For more information on Frequently asked question in Staining techniques, click here
Staphylococcus aureus: Abscess
What are pyogenic bacteria?
These are bacteria that induce pus formation upon infection.
Name some pyogenic bacteria.
Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Hemophilus influenzae etc.
How is the specimen collected from abscesses?
If the abscess is closed, pus can be collected in a sterile container after incision and drainage. Alternatively, the pus can either be aspirated with needle and syringe especially if anaerobic cultures are to be performed. In case of open abscess, pus can collected from the depth of the lesion after superficial cleaning. The material collected (pus) in swab must be kept in a sterile test tube and transported immediately to the laboratory.
Which are the investigations performed?
A smear is prepared on slide and stained with Gram's stain. The specimen is inoculated into Blood agar and incubated at 37oC overnight. Following identification of the pathogen, antibiotic susceptibility of the isolate is performed.
What is the Gram stain observation?
Plenty of pus cells along with Gram positive cocci in typical grape like clusters seen. This observation is consistent with the morphology of Staphylococcus.
Describe the growth seen on Blood agar.
Smooth, circular, convex, butyrous, golden-yellow pigmented colonies that are beta-hemolytic are seen.
Which is the other beta hemolytic bacterium?
Beta hemolytic Streptococci
Which are the other pigmented bacteria?
Psedomonas aeruginosa, Micrococcus luteus, Serratia marcesens, Chromobacterium violaceum and some chromogenic Mycobacteria produce pigmented colonies.
How do you identify this bacterium?
A Gram stained smear showing gram positive cocci in grape-like clusters suggests Staphylococcus. Further, a positive catalase test differentiates it from Streptococci. Positive mannitol fermentation and positive tube coagulase test confirms it as Staphylococcus aureus.
What is the significance of coagulase test?
Coagulase test is a pathogenicity test. It helps to identify the pathogenic species of Staphylococcus, namely S. aureus.
Which are the different types of coagulase test?
There are two types of coagulase test: slide coagulase and tube coagulase. Slide coagulase test is useful in detecting bound coagulase while tube coagulase test is useful in detecting free coagulase. While slide coagulase test is used for screening isolates, tube colagulase is used for confirmation.
How is slide coagulase test performed?
Two milky suspensions of the test isolate in saline are made on either side of a glass slide. One is marked as test and the other as control. To the suspension marked test, a loopful of undiluted rabbit plasma is added and mixed well. Immediate appearance of clumps indicates a positive slide coagulase test. The suspension marked control should not show any auto-agglutination.
How is tube coagulase performed?
Three test tubes are taken and marked test, positive control and negative control. Around 0.5 ml of overnight growth of test bacteria in broth is taken in test tube labeled test, 0.5 ml of known S. aureus suspension in test labeled positive and 0.5 ml of sterile broth is taken in tube labeled negative. To the tubes labeled test and positive 0.5 ml of 1 in 10 diluted rabbit plasma is added while same volume of sterile broth is added to tube labeled negative. The tubes are incubated at 37°C and observed at hourly interval for clotting of plasma. A positive test in indicated by clotting (gelling) of plasma, which does not flow when the tube is inverted. There should be no gelling in tube labeled negative.
Are there any other specific tests to identify S. aureus?
Other tests such as phosphatase test and tellurite reduction do help in identifying S. aureus but they are not specific. DNase test or thermonuclease test is more specific.
Which are the other infections produced by this isolate?
S. aureus is known to produce superficial infections such as boils, folliculitis, carbuncle, furuncle, stye and wound infections. They are responsible for deep infections like pharyngitis (less common), post-viral pneumonia, endocarditis, pyelonephritis, bacteremia etc. the toxin mediated diseases include food-poisoning, Toxic shock syndrome and Scalded skin syndrome. They are important nosocomial pathogens.
In what way the superficial lesions produced by S. aureus differ from those produced by Streptococci?
Streptococcal lesions tend to be spreading (due to hyaluronidase) while lesions produced by S. aureus tend to be limiting. The pus in streptococcal lesions tends to be more serous (due to breakdown of DNA by DNase) than in Staphylococcal lesions.
Which are the important virulence factors of S. aureus?
S. aureus is known to produce various surface factors, toxins and enzymes that helps in its pathogenicity. Important enzymes are coagulase, fibrinolysin, lipase, hyaluronidase, and DNase. Important toxins are hemolysins, enterotoxins, TSST-1 and exfoliative toxin. Cell wall peptidoglycan and teichoic acid too confer some degree of virulence.
How is this condition treated?
After the pus is drained, the patient must be put on suitable antibiotics. The choice of antibiotics depends on the results of antibiotic susceptibility test.
What is MRSA?
It stands for methicillin resistant S. aureus. An altered penicillin binding protein (PBP), which has low affinity to penicillins, renders them resistant to penicillinase resistant penicillins such as methicillin, oxacillin and nafcillin. These strains are resistant to all penicillins and cephalosporins and are multi-drug resistant. Serious infections by MRSA strains are currently treated using vancomycin. They are important nosocomial pathogens these days.
How are Staphylococci resistant to penicillins?
Majority of Staphylococci are resistant to penicillins owing to the production of penicillinase (also known as beta-lactamase) enzymes that inactivate penicillins. Other mechanism includes production of altered penicillin binding protein (PBP) with reduced affinity to the penicillins. This mechanism is seen in MRSA. Low-level resistance to vancomycin too has emerged among these isolates.
How is Staphylococcus classified?
Staphylococcus were initially classified on the basis of their pigment production into three: S. aureus (golden yellow pigment), S. citreus (lemon yellow pigment) and S. albus (non-pigmented; white). Since pigment production is not consistent and certain strains of S. aureus may not be pigmented, this system is not of much use. Practical way of classifying Staphylococcus is by way of coagulase test; coagulase positive isolates are usually S. aureus whereas coagulase negative isolates are called coagulase negative Staphylococcus.
What are coagulase negative Staphylococci?
Species of Staphylococcus other than aureus, which are coagulase negative are called coagulase negative Staphylococcus (CoNS). These reside as commensal on human or animal skin and were regarded as contaminants. Its role in human infections is increasing, especially as a nosocomial pathogen. S. epidermidis, S. saprophyticus, and S. intermedius are some of the CoNS. Formation of biofilms help some species to colonise readily. S. saprophyticus is known to cause UTI among sexually active young women ("honeymoon cystitis"). It is identified by its characteristic resitance to novobiocin (disk). Methicillin resistance also occurs among CoNS.
How are hospital strains identified?
Most of the hospital strains are resistant to multiple antibiotics. Healthcare-acquired methicillin-resistant Staphylococcus aureus, or HA-MRSA strains are mostly positive for Panton Valentine gene. Strains causing outbreaks in the hospital can be typed by several phenotypic and genotypic methods. One of the phenotypic methods include phage typing.
Streptococcus pyogenes: Pharyngitis
A 12 year old girl with high fever reports to hospital ENT OPD with complaint of severe sore throat and difficulty in swallowing food. On clinical examination the pharynx was inflamed with yellowish discharge were over the tonsils.
What is your diagnosis?
It is a case of acute pharyngitis or sore throat.
What are the etiological agents of pharyngitis?
Pharyngitis can be caused by bacteria such as Group A Streptococci, Staphylococcus aureus, Corynebacterium diphtheriae, fusobacterium-treponemes or by viruses such as Herpes Simplex, Epstein Barr virus or even fungus such as Candia albicans.
How is the specimen collected?
Two sets of throat swabs are collected by rubbing over the inflamed area in throat and tonsils. Care should be taken not to touch the tongue, cheeks or palate.
Which transport medium can be used in case of delay?
Pike's medium
How is the specimen processed?
One swab is used for preparing a smear and stained by Gram stain. This will give information on possible etiology. Presence of Corynebacterium diphtheriae can be ruled out by Albert's stain. The other swab is used to inoculate on blood agar and incubated at 37oC aerobically or in candle jar overnight. Rapid detection of Streptococcus antigens from the throat swab extracts is now possible using latex agglutination, co-agglutination, Enzyme immunoassay and immunoblot techniques.
How do you identify the growth?
Small, circular, non-pigmented greyish colonies with wide zone of beta hemolysis are seen on Blood agar. Gram stained smear will demonstrate gram positive cocci in chains. A negative catalase test will confirm it as Streptococci. Further identification of the species can be made by Lancefield typing of the isolate. Streptococcus pyogenes reacts with group A antiserum. S. pyogenes is also identified by positive PYR test.
Which are the possible complications of this condition?
Infection may spread by extension locally and result in tonsillar abscess, retropharyngeal abscess , mastoiditis, sinusitis, or otitis media. It may also lead to post streptococcal sequelae.
How would you treat this condition?
S. pyogenes is susceptible to penicillin and cepahlosporins. Patients allergic to penicllin may be treated by other antibiotics such as erythromycin.
Which are the other lesions produced by beta hemolytic streptococci?
Beta hemolytic streptococci (Group A) are responsible for scarlet fever, pyoderma (impetigo), erysipelas, cellulitis, myositis, puerperal sepsis, wound infections, etc. Important post-streptococcal sequelae include Acute rheumatic fever (ARF) and acute glomerulonephritis (AGN).
What is the pathogenesis of ARF?
Recurrent attacks of pharyngitis by Group A Streptococci may lead to ARF. Some degree of antigenic cross-reactions is known to occur between human antigens and streptococcal antigens. An immune response against the bacterial antigens is thought to mediate an attack on cross-reacting self-antigens. Some amount of genetic predisposition is also known to occur in such patients. Certain rheumatogenic strains belonging to M serotypes 1, 3, 5, 6 and 18 are frequently associated with ARF.
How is the diagnosis of ARF made using laboratory tests?
A culture from throat swab is not useful as this condition sets in after an episode of pharyngitis. A retrospective diagnosis can be made serologically by detecting anti-streptolysin O antibodies in patient's serum. The ASO test that is frequently used is based on latex agglutination. A titre of 200 units or higher is considered significant.
Why is anti-streptolysin S not used?
Streptolysin S is not antigenic; hence there are no anti-streptolysin S antibodies.
What is the pathogenesis of AGN?
AGN may follow either pharyngitis or pyoderma by Group A Streptococci. The antibodies that are formed in the body towards streptococcal antigens form antigen-antibody complexes. These complexes get deposited in the glomerular basement membrane of the kidney and elicit complement-mediated attack. Complement levels are usually low in AGN. Certain nephritogenic strains belonging to serotype 1, 4, 6, 12, 25 are associated with pharyngitis-associated AGN whereas serotypes 2, 49, 53, 55, 56, 57, 60 are associated with pyoderma-associated AGN.
How is the diagnosis of AGN made with laboratory tests?
ASO tests are usually not positive in most cases of AGN. Streptodornase test (anti-DNase B) and anti-hyaluronidase tests are more helpful. A titre of 300-350 units or higher of anti-DNase B is considered significant. Streptozyme, a passive hemagglutination test using crude extracts of Streptococci is positive in both AGN and ARF.
Streptococcus pneumoniae: Lobar pneumonia
A 46 year old febrile man was admitted to the hospital. He had been coughing with yellowish expectoration. Physical examination revealed high fever (38°C), tachycardia, tachypnea and appeared confused. He also complained of chest pain. Breath sound appear crackled. Chest X-ray suggested dense left lower lobe consolidation. Hematological examination revealed leucocytosis and CRP was elevated.
What is your diagnosis?
It is a case of acute lower respiratory tract infection, probably lobar pneumonia. Differential diagnosis must include viral or fungal pneumonia, bronchitis, COPD, lung abscess etc.
Which are the bacterial etiological agents of pneumonia?
Pneumonia can be caused by bacteria such as Streptococcus pneumoniae (pneumococcus), Hemophilus influenzae, Klebsiella pneumoniae, Staphylococcus aureus. Other rarer pathogenic bacteria include Legionella pneumophila and Pseudomonas aeruginosa. People with poor oral hygiene, altered swallowing reflexes, or impaired consciousness are predisposed to infection by anaerobes due to aspiration of oral fluids. Mycoplasma pneumoniae is known to cause primary atypical pneumonia.
What are the specimens collected?
Patient is asked to expectorate sputum into a sterile container. In a severely ill patient specimen such as bronchial washing specimen or transtracheal aspirate may be taken. Blood may be collected for blood culture, CRP & routine blood examination. Urine may also be collected for demonstration of pneumococcal antigens.
How is the sputum specimen processed?
A gram stained smear should be made from the thick part of the sputum and observed for pus cells and bacteria. A good specimen must have less than 10 squamous epithelial cells and more than 25 pus cells per low-power field. The sputum should also be inoculated on to Blood agar or chocolate agar and incubated at 37oC in 5-10% CO2, preferably in a candle jar.
What are your observations?
Gram smear of sputum showed plenty of pus cells along with gram positive lanceolate shaped cocci in pairs. Small, circular, smooth, draughtsman-type colonies with alpha hemolysis were seen on blood/chocolate agar.
How do you identify the growth?
Gram stained smear of the colonies revealed gram positive lanceolate shaped cocci in pairs suggestive of pneumococci. These colonies were catalase negative. Bile solubility, inulin fermentation, optochin susceptibility, quellung reaction and mouse intraperitoneal inoculation may be done to differentiate pneumococci from viridans streptococci. Pneumococci are positive for bile solubility, inulin fermentation, quellung reaction, are susceptible to optochin and are pathogenic to mouse. The capsular antgien may be detected by latex agglutination or co-agglutination.
What is quellung reaction?
The Neufeld's quellung reaction is also known as "capsule swelling" reaction. When a suspension of pneumococcal colonies are treated with a loop of serum containing anitbodies to capsular polysaccharide and observed under microscope, the capsule appears swollen. The binding of antibodies to capsular antigen brings about a change in its refractive index, making it appear swollen. A drop of methylene blue may also be added to the suspension to provide contrast. The serum used may be monovalent or polyvalent (omniserum).
What is optochin susceptibility?
Optochin is ethyl hydrocuprein hydrochloride, a disc 5 µg of strength is placed on the lawn culture of pneumococci and incubated. A wide zone of inhibition (at least 10-13 mm diameter) around the disc indicates susceptibility.
What is bile solubility test?
Pneumococci have amidase enzymes that result in autolysis. These enzymes can be activated by surface active agents such as bile salts. Bile solubility test can be done in test tube or in culture plates. To a turbid, 1ml overnight broth culture of pneumococci, addition of few drops of 10% sodium deoxycholate results in clearance of the broth in 15 minutes. Colonies suspected to be of pneumococci are marked and a loopful of 2% sodium deoxycholate is placed on them and incubated at 37oC for 30 minutes. The disappearance of colonies leaving behind an area of alpha hemolysis indicates positive test.
What is the pathogenesis of pneumococcal pneumonia?
Pneumonia is defined as inflammation and consolidation of the lung tissue due to an infectious agent. Streptococcus pneumoniae reach the lungs after first colonizing the oropharynx. S pneumoniae generally resides in the nasopharynx and is carried asymptomatically in approximately 50% of healthy individuals. Viral infections increase pneumococcal attachment to the receptors on activated respiratory epithelium. Presence of capsule is a major virulence factor as it helps the bacterium to evade phagocytosis. The pneumonic lesion progresses as pneumococci multiply in the alveolus and invade alveolar epithelium. Pneumococci spread from alveolus to alveolus, thereby producing inflammation and consolidation along lobar compartments. A patchy bronchopneumonic pattern involving the lower lobes is seen in the elderly. Since S. pneumoniae infection has a tendency to involve the pleura, pleural effusion is often seen.
Which are the other infections produced by pneumococci?
Pneumococci is known to cause sinusitis, otitis media, bronchitis, lung abscess, septic arthritis, septicemia, meningitis, peritonitis and endocarditis.
Which are the antibiotics used in the treatment of this condition?
Penicillin used to be the drug of choice but large number of strains are now developing resistance due to alteration in the penicillin binding proteins. Alternate choices include macrolides (erythromycin, roxithromycin, clindamycin), quinolones (ciprofloxacin, levofloxacin) , cephalosporins (cefuroxime, cefpodoxime, cefotaxime). Many of the penicillin-resistant strains are also resistant to erythromycin, cotrimoxazole, tetracycline, and chloramphenicol. The choice of suitable antibiotic must be made after antibiotic susceptibility testing only.
Which conditions can predispose to pneumococcal infections?
Chronic alcoholism, splenectomy, previous viral respiratory illness, malnutrition, chronic smoking, cirrhosis of liver, coronary artery disease etc.
Are any vaccines available against pneumococcal disease?
A 23-valent polysaccharide vaccine against 23 common serotypes has been in use in some countries. 23-valent pneumococcal polysaccharide vaccines has been recommended for use among children aged ≥2 years who have high rates of disease, including those with sickle cell disease (SCD), chronic underlying diseases, human immunodeficiency virus (HIV) infection, or others who are immunocompromised. 23-valent pneumococcal polysaccharide vaccines are effective in preventing invasive pneumococcal disease among older children and adults, these vaccines do not protect children aged <2 years. A 7-valent pneumococcal polysaccharide-protein conjugate vaccine was licensed for use among infants and young children as it decreases colonization and prevents pneumococcal disease among children aged ≤2 years.
Mycobacteriumn tuberculosis: pulmonary tuberculosis
A 55 year old man with persistent cough, fever, night sweats, loss of weight, anorexia, malaise and weakness since 3 months presents himself to the hospital. Chest X-raysuggested upper lobe consolidation. Hematological examination revealed mild leucocytosis.
What is your diagnosis?
It could be a case of pulmonary tuberculosis; however differential diagnosis includes aspergillosis, actinomycosis, bronchiectasis, histoplasmosis, lung abscess, blastomycosis etc.
What is the etiological agent of tuberculosis?
Typical pulmonary tuberculosis is caused by Mycobacterium tuberculosis, however other species of Mycobacteria such as M. bovis, M.avium-intercellulare complex too can cause tuberculosis.
What is the specimen collected?
Expectorated sputum specimen is the preferred specimen in the laboratory diagnosis of pulmonary tuberculosis. According to the revised guidelines of the National Tuberculosis Control Programme (India), two sputa samples (spot-morning) must be collected from the patient. When the patient approaches the center to collect the container, spot specimen is collected. Patient expectorates the second sample on the next morning in the given container and submits it to the laboratory. After rinsing the mouth, patient should take deep breath, cough and expel the sputum into the container that is held close to the mouth. In patients without spontaneous sputum production, sputum induction may be induced using hypertonic saline.
Which are the other specimen that can be obtained from patients?
In case of children (who tend to swallow sputum), a morning gastric lavage may be collected. Alternatively laryngeal swabs may also be collected. Other invasive techniques that can be employed include fiberoptic bronchoscopy with transbronchial biopsy, bronchial brushings, and transtracheal aspirate.
How is the specimen processed?
A gram stained smear may be made from the thick part of the sputum to exclude other bacterial infection. Acid fast staining of the smear is considered the gold standard in the laboratory diagnosis. After assessing the suitability of the sample, the smear is stained with any of the acid fast staining techniques (Ziehl Neelsen, Kinyoun, Gabbett). Hundred fields must be observed in each smear before giving a negative report. If acid fast bacilli are seen, they should be counted and the smear is graded.
What are your observations?
Pink coloured, slightly curved bacilli in singles or clumps, with occasional branching and beaded appearance are seen against a blue background consisting of many pus cells and few epithelial cells. The given smear contains acid fast bacilli.
How is sputum graded?
According to the RNTCP, sputum sample should be graded in the following way:
>10 AFB/oil immersion field in at least 20 fields: 3+
1-10 AFB/oil immersion field in at least 50 fields: 2+
10-99 AFB/100 oil immersion field: 1+
1-9 AFB/100 oil immersion field: record exact number
What is the significance of grading the sputum smear?
The initial count gives a picture of the extent of the disease. It has a very important role in monitoring the progress of treatment, as the counts decrease with successful treatment.
What is the sensitivity of sputum smears?
AFB smear is not a very sensitive technique, if numbers of bacilli are less than 1000/ml of sputum, they may be missed. The sensitivity of the smear can be increased by subjecting the sample to concentration techniques such as Petroff's method, Cetyl pyridinium chloride, Zepharin chloride method, or NALC method etc. The sensitivity of microscopic examination of sputum can be increased by using fluorescent dyes such as Auramine O.
How is the diagnosis made using sputum smears?
The following is the recommendation of RNTCP on patient with cough for more than two weeks: If one or two samples of sputa are smear positive it may be considered as sputum smear-positive tuberculosis and the patient is put on anti-tuberculosis treatment. If neither smear is positive but X-ray findings are suggestive of TB, the patient is considered smear-negative and put on treatment. If the x-ray is not suggestive, then TB is ruled out. Tuberculosis is ruled out if all the smears are negative and x-ray finding too is not suggestive. See the algorithm here.
How are Mycobacteria cultured?
Direct sputum sample (or sputum concentrate) is usually cultured on Lowenstein Jensen Medium and incubated at 37oC for 4-8 weeks. Cultures are not routinely performed, but may be done to identify the species or for drug susceptibility testing. Mycobacterium tuberculosis produces rough, buff and tough colonies on LJ medium. The acid fast smear of these colonies show acid fast bacilli. Conventional culture media used for Mycobacterial isolation are most often Lowenstein-Jensen (LJ), or Middlebrook 7H9, 7H10 or 7H11.Growth in these media is observed in a range of 3 – 56 days, depending on the species isolated and concentration of viable bacteria. The SEPTI-CHEK AFB system is intended for use as an integrated in-vitro diagnostic system for the detection and isolation of Mycobacteria from various clinical specimens, which provides a presumptive identification as well as the ability to perform susceptibility testing. In miliary tuberculosis, Mycobacteria can be recovered from blood using BACTEC-460 TB system; a system employing radiometric technology providing rapid and accurate detection in as little as 4-8 days and susceptibility testing in as little as 4-12 days. Other system includes Mycobacterial growth indicator tube (MGIT) system. Culture using animal models are no longer employed for routine diagnosis.
How is Mycobacterium tuberculosis identified?
Conventional method of identification are aryl sulfatase test, niacin test, nitrate reduction, thermocatalase test etc. High pressure liquid chromatography for detection of mycolic acid, nucleic acid hybridization, PCR have replaced the conventional system in developed countries.
Which are the other investigation techniques employed in the laboratory diagnosis of tuberculosis?
Serological methods detecting mycobacterial antigens or IgA, IgG or IgM antibodies against Mycobacterium using ELISA have been employed but have not met with great success. Skin testing (tuberculin test such as PPD, Mantoux) have been employed to test for sensitivity to Mycobacteria. Reading the test has been prone to error and are subjected to many false positive and negative results.
What is tuberculin test?
Please read this notes.
What is the pathogenesis of tuberculosis?
Tuberculosis (TB) is spread from person to person through the air by droplet nuclei that contain M. tuberculosis. Droplet nuclei are produced when persons with pulmonary tuberculosis cough, sneeze, or speak.Infectious dose is less and few bacilli can cause infection. Droplet nuclei are small enough to reach the alveoli within the lungs. Alveolar macrophages ingest the bacilli and enclose them in phagosomes. If these macrophages are activated, the mycobacteria containing phagosomes fuse with lysosomes, and the bacteria are killed. If, on the other hand, the alveolar macrophages are not activated, the bacilli survive and multiply within the phagosomes. The macrophages lyse and the mycobacteria are released into the surrounding lung tissue, where they are phagocytized by tissue macrophages. Again, if the macrophages are activated, the bacteria are killed. However, if these tissue macrophages are not activated, the mycobacteria continue to multiply within the phagosomes and, upon release, are phagocytized by additional tissue macrophages and the infection spreads. As this process continues, a primary lesion forms. As the primary lesion enlarges, some mycobacteria are transported to the regional draining lymph nodes and the lymph nodes enlarge as the bacilli multiply intracellularly. Extension from the lung parenchyma or the lymph nodes lead to progressive primary tuberculosis. They may also become dormant and remain asymptomatic, or may proliferate after a latency period (reactivation disease). The main determinant of the pathogenicity of tuberculosis is its ability to escape host defense mechanisms, including macrophages. Among the several virulence factors in the mycobacterial cell wall are the cord factor, lipoarabinomannan, and a 65-kd heat shock protein. Progression of the primary complex may lead to enlargement of hilar and mediastinal nodes. Lymphohematogenous dissemination of the mycobacteria to other body parts and their multiplication results in miliary or disseminated tuberculosis. Tubercular meningitis may also result from hematogenous dissemination. Bacilli may remain dormant in the apical posterior areas of the lung for several months or years, which may later progress resulting in the development of reactivation-type tuberculosis.
How is tuberculosis treated?
Directly observed short-course chemotherapy for newly diagnosed cases and sputum smear negative but seriously ill with tuberculosis are subjected to intensive phase treatment regimen comprising of isoniazid, rifampicin, pyrazinamide and ethambutol that is administered three times a week for two months. When the patient has completed the initial intensive phage of two months and the sputum smear becomes negative, the continuation phase is begun. If the sputum remains positive despite the intensive phase, then the four drugs of intensive phase is continued for another month. After this period, continuation phase is begun irrespective of smear status. The continuation phase consists of isoniazid and rifampicin given three times a week for four months.
Why are anti-tubercular drugs given in combination?
Spontaneous mutation can result in development of resistant strains even during the course of treatment. Inclusion of more than one drug ensures that strains resistant to one drug are killed by the other drug.
What are MDR-TB and XDR-TB?
Please read this notes.
Are there any rapid commercial methods of diagnosis?
Interferon-Gamma Release Assays (IGRAs) measure a person’s immune reactivity to M. tuberculosis. Freshly drawn patient's blood samples are mixed with purified M. tuberculosis antigen (synthetic peptides). White blood cells of the patient that are reactive to M.tuberculosis will release interferon-gamma, which is quantitatively measured. Results of this test can be available within 24 hours. However, this test does not help in differentiating latent tuberculosis infection from tuberculosis disease. Prior vaccination with BCG does not cause a false-positive result.
How can antibiotic resistance be detected in short time?
Line probe assay is a PCR and DNA hybridization based molecular tool that can detect resistance to rifampin and isoniazid. This test can be performed directly on smear-positive sputum samples and results would be available in five hours.
Neisseria meningitidis: meningitis
A 10 year old child is brought to the hospital with high fever, neck rigidity, and petechial rashes on the body. The child had complained of severe headache and had vomited before being brought in. Photophobia and an altered mental status was also noted. Physical examination showed Kernig's and Brudzinski's signs to be positive.
What is your diagnosis?
It could be a case of acute pyogenic meningitis with septicemia, probably meningococcemia with meningitis.
Which are the bacteria that can cause pyogenic meningitis?
Neisseria meningitidis, Streptococcus pneumoniae, Escherichia coli, Hemophilus influenzae, Streptococcus agalactiae and Listeria monocytogenes can cause acute bacterial meningitis.
What is meningitis?
Meningitis is the term to denote inflammation of the meninges. Depending on the duration of symptoms, meningitis may be classified as acute (hours to days) or chronic (weeks to months). Acute bacterial meningitis is caused by bacteria and is characterized by an acute onset of meningeal symptoms and neutrophilic pleocytosis. Aseptic meningitis characteristically have an acute onset of meningeal symptoms and cerebrospinal pleocytosis that is usually prominently lymphocytic. While viruses cause most cases of aseptic meningitis, it can also be caused by bacterial, fungal, mycobacterial, and parasitic agents.
What is the pathogenesis of meningitis?
Initially, the infectious agent colonizes or establishes a localized infection in the host. This may be in the form of colonization or infection of the skin, nasopharynx, respiratory tract, gastrointestinal tract, or genitourinary tract. Most meningeal pathogens are transmitted through the respiratory route. Both N. meningitidis and S. pneumoniae are known to colonize the oropharynx. From this site, the organism gains access to the CNS by invasion of the bloodstream and subsequent hematogenous seeding of the CNS, a retrograde neuronal (ie, olfactory and peripheral nerves) pathway or direct contiguous spread (ie, sinusitis, otitis media). Once inside the CNS, the infectious agents likely survive because host defenses (eg, immunoglobulins, neutrophils, complement components) appear to be limited. Inflammation of meninges is initiated by the presence of several bacterial components such as lipopolysaccharide and teichoic acid in the subarachnoid space. These components stimulates monocytes and macrophages to produce cytokines such as TNF-α, IL-1 and IL-8. The inflammatory response elicited by these cytokines are responsible for clinical manifestations of meningitis. The fundamental pathologic change in meningococcemia is widespread vascular injury characterized by endothelial necrosis, intraluminal thrombosis, and perivascular hemorrhage. Skin lesions usually contain numerous meningococci undergoing phagocytosis by neutrophils.
How is this condition diagnosed using laboratory techniques?
Since there is both meningitis and septicemia, both blood as well as CSF must be collected. Laboratory diagnosis involves microscopic examination of CSF smear, culture from blood & CSF as well as detection of bacterial antigen.
Which are the specimens collected?
Approximately 3-5 ml Spinal fluid (CSF) is collected by spinal tap (lumbar puncture) by inserting the needle between L3 and L4 vertebrae into a sterile container. Only 3-5 ml of CSF must be collected and the rate of collection should be slow(4-5 drops/second). Alternatively, 1 ml fluid each may be collected in three separate containers. Five ml of venous blood may be drawn by venipuncture for blood culture.
Which are the necessary investigations performed?
CSF should be subjected to cytological (cell type & cell count), biochemical (glucose & protein level) and microbiological investigations. In bacterial meningitis CSF is usually purulent (>100 cells/mm2), containing polymorphonuclear leucocytes and the glucose level is usually less than half the serum level. Microbiological investigations include microscopy (Gram stained smear), antigen detection and culture followed by antibiotic susceptibility testing. If the CSF is clear, it should be centrifuged and the deposit taken for microscopy and culture. A loopful of CSF is inoculated on to Blood agar and Chocolate agar and incubated at 37oC in the presence of 5-10% CO2 in a candle jar and incubated overnight. CSF may be subjected to antigen detection by latex agglutination, co-agglutination or counterimmuno-electrophoresis. The blood may be subjected to antigen detection and culture. Blood is inoculated into brain heart infusion broth and incubated at 37oC, it is then subcultured to Blood agar the following day.
What are your observations?
The gram stained smear shows plenty of pus cells and gram negative diplococci (both intracellular and extracellular). Round, smooth, moist, glistening, convex, greyish and unpigmented colonies with an entire edge that may be 1-4 mm wide are seen on blood agar. Older cultures may sometimes cause the underlying agar to turn dark. The gram stain of the colonies display gram negative cocci in pairs. These colonies are oxidase positive. Similar colonies are obtained from subculture of blood. The isolate fermented glucose and maltose but not lactose or sucrose. The isolate is identified as Neisseria meningitidis. Latex agglutination test for Neisseria antigen in CSF and blood was also positive.
E.coli: urinary tract infection
A 23 old female complains of mild fever, increased frequency, urgency and burning micturition. She also reported a sensation of bladder fullness, lower abdominal discomfort and flank pain.
What is your diagnosis?
It could be a case of urinary tract infection. Some of the differential diagnoses include urethritis, PID, endometriosis, vaginitis and renal calculi.
Which are the bacteria that can cause urinary tract infection?
Common uropathogens of community acquire UTI include E.coli, Klebsiella pneumoniae, Proteus sps, and Enterobacter sps. In hospitalized patient, who have a urinary catheter, Pseudomonas sps, and Enterococci are common pathogens. Staphylococcus saprophyticus is known to cause UTI in sexually active young women.
What is the common source of UTI?
In community acquired UTI, the uropathogens frequently are one's own enteric flora. UTI is more common in women than men due to proximity of anus to vagina and shorter urethra.
How are urinary tract infections classified?
UTI may be community acquired or hospital acquired, lower or upper, ascending or descending, uncomplicated or complicated.
How is the sample collected for laboratory diagnosis?
An early morning, freshly voided, clean-catch, mid-stream urine should be collected in a sterile, wide mouthed container after proper anogenital toilette. The external genitilia must be cleansed with mild antiseptic or soap before sample collection to avoid contamination of the urine by normal flora present in this region. In men, the prepuce is retracted and in women, the labia is spread apart and then the middle portion of the urine is collected in the container. The sample must be labeled and sent to the laboratory without delay.
Which are the other techniques to collect urine specimen?
In infants urine flow may be stimulated by tapping just above the pubis with two fingers at one hour after a feed. One tap per second is given for one minute and after an interval of one minute tapping is continued. Under certain conditions, suprapubic aspiration of urine directly from the bladder may be performed. Since this is an invasive technique, it must be performed only when absolutely necessary. Catheterization only for the purpose of collecting urine should be avoided as it may induce infection. In situations where the patient is already catheterized, the urine must not be collected from the bag, instead, it should be aspirated from catheter tube using needle and syringe.
How long can the urine be held before testing?
Ideally, urine must be processed as soon as possible since urine supports growth of bacteria. In case of delay of 1-2 hours the sample may be refrigerated or treated with boric acid at an concentration of 1.8%. Another way of preserving the sample in case of delay is by collecting urine in sterile vacutainer tubes containing boric acid-sodium formate transport medium. Samples that have been processed after a delay of five hours or more do not give reliable results.
Which investigations are performed on urine sample?
Urine wet mount and culture is commonly performed on urine specimen. Wet mount examination is performed to look for pus cells, RBCs and casts. A loopful of well mixed urine placed on the glass slide (without spreading) can be stained by Gram stain and observed. Presence of single bacterium per oil immersion field in such a smear indicates significant bacteriuria. Screening test such as nitrate reduction, dipstick, tetrazolium reduction etc are not specific and are not routinely done. Leukocyte esterase dip test is helpful in detecting pyuria. Qualitative culture technique such as Miles and Misra are too cumbersome to perform for routine diagnosis, hence a semi-quantitative culture is performed by calibrated loop method. A loopful of well-mixed uncentrifuged urine is inoculated on to CLED agar/MacConkey agar and Blood agar without sterilizing the loop in between.
What is significant pyuria?
Presence of at least 1000 pus cells per ml of uncentrifuged urine is significant pyuria. Ordinarily, presence of ≥10 pus cells/HPF in centrifuged urine and ≥5 pus cells in uncentrifuged urine is considered significant. Some authors consider counts as low as 2-5 WBCs /HPF important in a centrifuged specimen in the female with appropriate symptoms. In women, contamination from vagina may introduce large numbers of pus cells into a sample of voided urine. The presence of squamous epithelial cells along with pus cells in the sample is evidence that contamination has occurred and the pus cell count is not significant.
What is significant bacteriuria?
Since normal voided urine tends to get contaminated with normal flora of the distal urethra, differentiation of contamination from urinary tract infection is made by quantifying the bacterial growth. Significant bacteriuria is a concept put forth by Kass EH, who stated that there should be at least 1,00,000 bacteria of single type per ml of urine. This count may not be applicable in all situations. Recent studies suggest that a count of 102 per ml in acutely symptomatic women and a count of 103 per ml in symptomatic men may be significant. Any growth obtained from urine collected via suprapubic aspiration is significant. Lower counts may be significant when S. aureus is the pathogen.
How is semi-quantitative culture performed?
A loopful of well-mixed uncentrifuged urine is inoculated on the agar medium without sterilizing the loop and incubated at 37oC overnight. Following incubation, the number of colonies of single type is counted. A bacteriological loop of 3 mm diameter approximately carries 0.001 ml of urine. If this amount of urine gives rise to at least 100 colonies then the numbers of bacteria present in 1 ml can be obtained by multiplying by 1000, i.e 1,00,000 per ml.
What is your observation?
Wet mount of urine shows plenty of pus cells and RBCs but few squamous epithelial cells. More than 100 colonies of pink coloured (lactose fermenting), smooth, low convex, circular colonies of a single type is seen on MacConkey's agar. Gram stained smear of the colony shows gram negative bacilli, hanging drop shows motile bacilli, and catalase test is positive. Results of biochemical reactions are positive indole test, negative urea hydrolysis, negative citrate utilization, positive MR test and negative VP test. TSI agar shows acid slant/acid butt with little gas but no H2S. The isolate identified as Escherichia coli was obtained in significant count.
What factors must be borne in mind while interpreting urine culture reports?
Urine in the bladder is sterile, small numbers of bacteria get into the urine from the distal part of urethra while voiding. In typical cystitis, most often urine culture are unimicrobial, recovery of more than one type of bacteria in urine indicates contamination. Bacterial counts in the range of 102-103 in the absence of pyuria and other symptoms usually indicates contamination. Rarely, mixed infection by more than one type can occur; in such situations a repeat culture with same results is reliable. Significant bacteriuria may not be applicable for suprapubic aspirated urine. Bacterial counts can be lower if the patient is on antibiotics or has consumed large amount of water before voiding urine. Bacterial counts can be higher if there is a long delay between urine collection and culture.
What is sterile pyuria?
Presence of plenty of pus cells in urine but lack of growth on culture is sterile pyuria. The reasons for this condition include recent administration of antibiotics, UTI by a fastidious/auxotropic/anaerobic bacteria, urethritis due to gonococci, non-gonococcal urethritis (due to Ureaoplasma, Chlamydia, Trichomonas, or viruses) or renal tuberculosis.
What is baceriuria without pyuria?
It is the presence of large numbers of bacteria in the urine but lack of significant numbers of pus cells. This condition is usually seen in pregnant women where there is retention of urine or when voided urine is held for a long time before culture.
How is this condition treated?
Trimethoprim-sulfamethoxazole for 3 days is considered the current standard therapy for bacterial cystitis. Fluoroquinolones such as norfloxacin also works well. Antibiotics should be selected on the basis of susceptibility testing of the isolate.
Shigella sps: dysentery
A 13-year old male patient was admitted to hospital with complaint of bloody diarrhea along with tenesmus, cramping abdominal pain and fever. The amount of feces passed was scanty. The mucus was blood tinged, feces was not foul smelling and was adherent to the container.
What is your diagnosis?
It could be a case of bacillary dysentery.
Which are the pathogens that can cause dysentery?
Among bacteria that can cause dysentery include Shigella sps and Enteroinvasive E. coli. Bloody diarrhea can be caused by Salmonella sps, Campylobacter sps, Enterohemorrhagic E.coli etc. Among protozoa, Entamoeba histolytica and Balantidium coli can cause dysentery. Differential diagnoses should also include Crohn Disease and Ulcerative Colitis.
What are the differences between bacillary and amoebic dysentery?
| Amoebic dysentery | Bacillary dysentry | |
| Number of motions per day | 6-8 | >10 |
| Amount of feces | copious | scanty |
| Odour | Offensive | odourless |
| Colour | Dark red | Bright red |
| Nature | Blood and mucus mixed with feces | Blood and mucus, little feces |
| pH | Acid | Alkaline |
| Consistency | Not adherent to container | Adherent to the bottom of container |
| RBCs | Clumps | Rouleaux |
| Macrophages | Very few | Large and numerous |
| Pus cells | Scanty | Many |
| Pyknotic bodies | Common | Nil |
| Charcot Leydon crystals | Present | Absent |
| Ghost cells | Nil | Numerous |
What is the pathogenesis of dysentery?
Shigellosis is basically an enteric disease and the transmission involves feco-oral route. Shigella is transmitted via food, fingers, fomites or flies. Ingestion of small numbers of bacilli (approximately 102) can initiate infection in a susceptible person. As few as 10 S. dysenteriae bacilli can cause clinical disease, whereas 100-200 bacilli are needed for S. sonnei or S. flexneri infection. Once they survive the gastric juices, they reach large intestine and penetrate the cells of the epithelial lining. Incubation period can be short (1-7 days) but can be very short in people with achlorhydria. Pathogenesis requires invasion and toxin production. The characteristic virulence factor is encoded on a large (220 kb) plasmid that is responsible for synthesis of polypeptides that cause cytotoxicity. Shigella also produce siderophores that are coded on plasmid can chelate iron from host cells from its protein-bound state. Shiga toxin (Stx) is not essential for virulence of S dysenteriae type 1 but contributes to the severity of dysentery. The chromosomally encoded enterotoxin are are potent cytotoxins that are responsible for many pathogenic features of Shigella infection. Lipopolysaccaharide plays an important role in resistance to nonspecific host defense encountered during tissue invasion. The bacilli multiplies in the epithelia and spreads laterally to adjacent tissue and penetrate lamina propria. Inflammatory response ensues which ultimately leads to capillary thrombosis and sloughing off of epithelium due to necrosis leaving behind superficial ulcers. Some strains produce enterotoxin (Shiga toxin) that has neurotoxic, cytotoxic and enterotoxic action. The accumulation of inflammatory cells leads to the formation of microabscesses, which spread and coalesce to form larger abscesses. Bleeding occurs from superficial ulcerations. Bacterial shedding usually ceases within 4 weeks of the onset of illness. Chronic carriers are extremely rare.
What is the specimen collected and how is the condition diagnosed with the aid of laboratory?
Freshly passed feces is collected in a sterile wide mouthed container, if no feces is available rectal swabs may also be taken. A saline wet mount may be performed to observe pus cells, RBCs and their arrangement, or presence of any parasitic forms. In case of delay, the feces may be transported in transport medium such as Sach's buffered glycerol saline. Direct gram stained smear or hanging drop preparation is not useful. Feces is inoculated on to MacConkey's agar, and on to selective media such as XLD (xylose-lysine-deoxycholate) agar, DCA (deoxycholate citrate agar) or SS (Salmonella-Shigella) Agar and incubated at 37oC overnight. The sample may also be inoculated into enrichment broth such as selenite F broth and later subcultured on to MacConkey's agar for subsequent overnight incubation. An enzyme immunoassay for Shiga toxin is used to detect S. dysenteriae type 1 in the stool.
What is your observation?
Pale (non-lactose fermenting), smooth, low convex, circular colonies of a single type is seen on MacConkey's agar. Gram stained smear of the colony shows gram negative bacilli, hanging drop shows non-motile bacilli, and catalase test is positive. Results of biochemical reactions are negative indole test, negative urea hydrolysis, negative citrate utilization, positive MR test and negative VP test. TSI agar shows alkaline slant/acid butt with no gas or H2S. The isolate ferments mannitol with acid production. The isolate is identified as Shigella sps other than S. dysenteriae, which does not ferment mannitol.
How do you identify the species?
Sigella dysenteriae (Group A) does not ferment mannitol while the rest three serogroups ferment. Shigella sonnei (group D) is a late lactose fermenter and can be identified by a positive ONPG test. It is difficult to differentiate S. flexineri (group B) from S. boydii (group C). Confirmation of their identities can be made by slide agglutination using specific antisera.
Is it necessary to identify the species?
Identity of the species is not important for treatment. S. dysenteriae is known to cause severe form of dysentery than other species because it produces shiga toxin. Species identification is also epidemiologically significant. it is believed that S. dysenteriae is common in underdeveloped countries, S. flexineri in developing countries and S. sonnei is developed countries.
How is Shigella classified?
Shigella are grouped into 4 species: Shigella dysenteriae, Shigella flexneri, Shigella boydii, and Shigella sonnei, also known as groups A, B, C, and D, respectively. Group A has 13 serotypes, group B has 6 serotypes, group C has 18 serotypes, and group D has 1 serotype.
Which are the pathogenicity tests?
Pathogenicity tests to demonstrate Shigella's invasiveness include Sereny's test (development of keratoconjunctivitis in guinea pig), penetration of HeLa/Hep-2 cells in vitro or congo red binding test.
What are the complications of Shigella dysentery?
Complications include bacteremia, hemolytic uremic syndrome (microangiopathic hemolytic anemia, thrombocytopenia, and renal failure), septic arthritis, toxic neuritis, conjunctivitis, intussusception in children, intestinal perforation, and septicemia.
Is antibiotic treatment necessary?
Mild cases of dysentery are self limiting, but in pediatric cases or severe dysentery antibiotic administration is necessary. Resistance of Shigella species to sulfonamides, tetracyclines, ampicillin, and trimethoprim-sulfamethoxazole (TMP-SMX) has been reported worldwide, and these agents are not recommended as empirical therapy. Since Shigella are acquiring resistance to multiple antibiotics, antibiotic susceptibility testing must be performed along with culture.
Vibrio cholerae: Cholera
Several students of a primary school in a village fell ill. All of them were admitted to local hospital following vomiting and diarrhea. Purging was effortless and the feces was non-odorant and rice-watery.
What is your diagnosis?
It is a case of gastoenteritis, probably cholera.
Which are the pathogens that can cause gastroenteritis?
Common bacteria that can cause gastroenteritis include vibrio cholerae and Enterotoxigenic E.coli (ETEC).
What is the pathogenesis of cholera?
Cholera is basically an enteric disease and the transmission involves feco-oral route. Vibio cholerae is transmitted following ingestion of contaminated food or water. Ingestion of large numbers of bacilli (approximately 106) can initiate infection in a susceptible person. Once they survive the gastric juices, they reach small intestine and colonize the epithelial lining and produce cholera toxin. They adhere to the intestinal wall mediated by toxin-coregulated pilus (TCP). Hemagglutinin/protease is a zinc-dependent protease, which cleaves the mucin
and fibronectin and may serve to facilitate the spread and excretion of vibrios within the intestine in stools by detaching them from the intestinal walls. Incubation period can be short (1-3 days). Cholera toxin is holotoxin consisting of A subunit and B subunit. The B subunit binds to specific ganglioside receptors (GM1) on the epithelial cells of small intestine and facilitates the entry of A subunit where it activates adenylate cyclase. Stimulation of adenylate cyclase causes an increased production of cAMP, which leads to hypersecretion of water and electrolytes into the lumen and inhibition of sodium reasborption.
What is the specimen collected and how is the condition diagnosed with the aid of laboratory?
Freshly passed feces is collected in a sterile wide mouthed container, if no feces is available rectal swabs may also be taken. In case of delay, the feces may be transported in transport medium such as Cary Blair medium or VR medium. The enrichment medium alkaline peptone water may also be used as transport medium. Direct gram stained smear and hanging drop preparation may be useful. Feces is inoculated on to MacConkey's agar, and on to selective media such as TCBS Agar and incubated at 37oC overnight. The sample may also be inoculated into enrichment broth such as alkaline peptone water and subcultured after 6 hours of incubation on to MacConkey's agar for subsequent overnight incubation.
What is your observation?
Hanging drop preparation or darkground microscopy shows actively motile (darting-type) bacilli. Vibrio can be detected by immobilization test, wherein addition of vibrio antisera renders the bacilli in feces non-motile. Gram stain of mucus flakes in feces shows several curved, gram negative bacilli. Pale (non-lactose fermenting), smooth, low convex, circular colonies is seen on MacConkey's agar. On further incubation, colonies turned pink due to late lactose fermentation. TCBS agar shows yellow coloured colonies. Surface pellicle is noticed in alkaline peptone water. Gram stained smear of the colony shows curved gram negative bacilli, hanging drop shows actively motile bacilli, catalase test and oxidase test are positive. Results of biochemical reactions are: positive indole test, negative urea hydrolysis, positive citrate utilization, positive MR test and negative VP test. TSI agar shows acid slant/acid butt with no gas or H2S. The isolate ferments mannose and sucrose with acid production. Other tests that help in identifying Vibrio are string test and nitrosoindole test. The isolate is identified as Vibrio cholerae.
How do you identify the species?
Slide agglutination with antiserum against H antigen confirms that it is a Vibrio sps. Further slide agglutination using O1 antiserum is used to identify V. cholerae O1 from NAG vibrios. Serotyping by slide agglutination is performed to identify Ogawa, Inaba or Hikojima serotypes. Biotyping is undertaken by performing chick RBC agglutination, Hemolysis, VP test, susceptibility to Polymyxin B and Phage IV to differentiate ElTor from Classical biotype.
Is it necessary to identify the serotype and biotype?
Identity of the serotype and biotype is not important for treatment. Serotyping and biotyping is only epidemiologically significant.
Is antibiotic treatment necessary?
The mainstay of treatment is replacement of water and electrolytic, restoration of acid-base balance; role of antibiotics is secondary and serves to eradicate bacteria.
What are the complications of cholera?
After dehydration, hypoglycemia is the most common lethal complication of cholera in children. Acidosis in cholera is a result of bicarbonate loss in stools and accumulation of lactate. Acidemia occurs when respiratory compensation is unable to sustain a normal blood pH. Hypokalemia results from potassium loss in the stool.